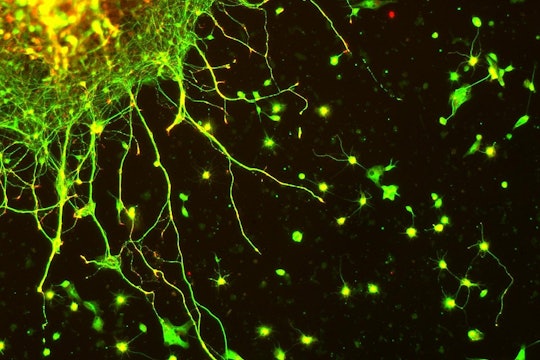
How scientists are mapping the building blocks of life
A microscopic moonshot hopes to revolutionize biology
From a single fertilized embryo, 37 trillion cells arose to form you, from the neurons in your brain, firing as you read these words, to the skin cells in your hands, touching a mouse, keyboard, or screen. A German scientist first proposed this notion in the mid-19th century, and not long afterward a Prussian biologist popularized the phrase omnis cellula e cellula, or “all cells come from cells,” cementing the idea that every living organism is made of cells.
Since then, textbooks have racked up 200 to 300 types of cells in your body – still only the tip of the cellular iceberg, seemingly. Recent genetic sequencing technology has led to the discovery of new types in the brain, gut, retina, and immune system, suggesting our knowledge of cells is more limited than we originally thought. Realizing the need and potential for an atlas of all human cells, two scientists, Aviv Regev and Sarah Teichmann, have set out to map every cell in the human body.
The cell is to biology what the atom is to chemists: the basic building block of the field. Despite this, biology’s “periodic table” is deeply incomplete. As Regev, of the Broad Institute of MIT and Harvard, began publicizing her efforts to complete the table, Teichmann, of the Wellcome Trust Sanger Institute, approached her to join forces. Not long afterward, the Human Cell Atlas Consortium (HCA) was conceived. With the inaugural meeting in October 2016, the Consortium, consisting of hundreds of scientists guided by a committee of 27 and $200 million in initial funding, recently announced data from the first million cells. They aim to genetically sequence and describe up to a trillion cells.
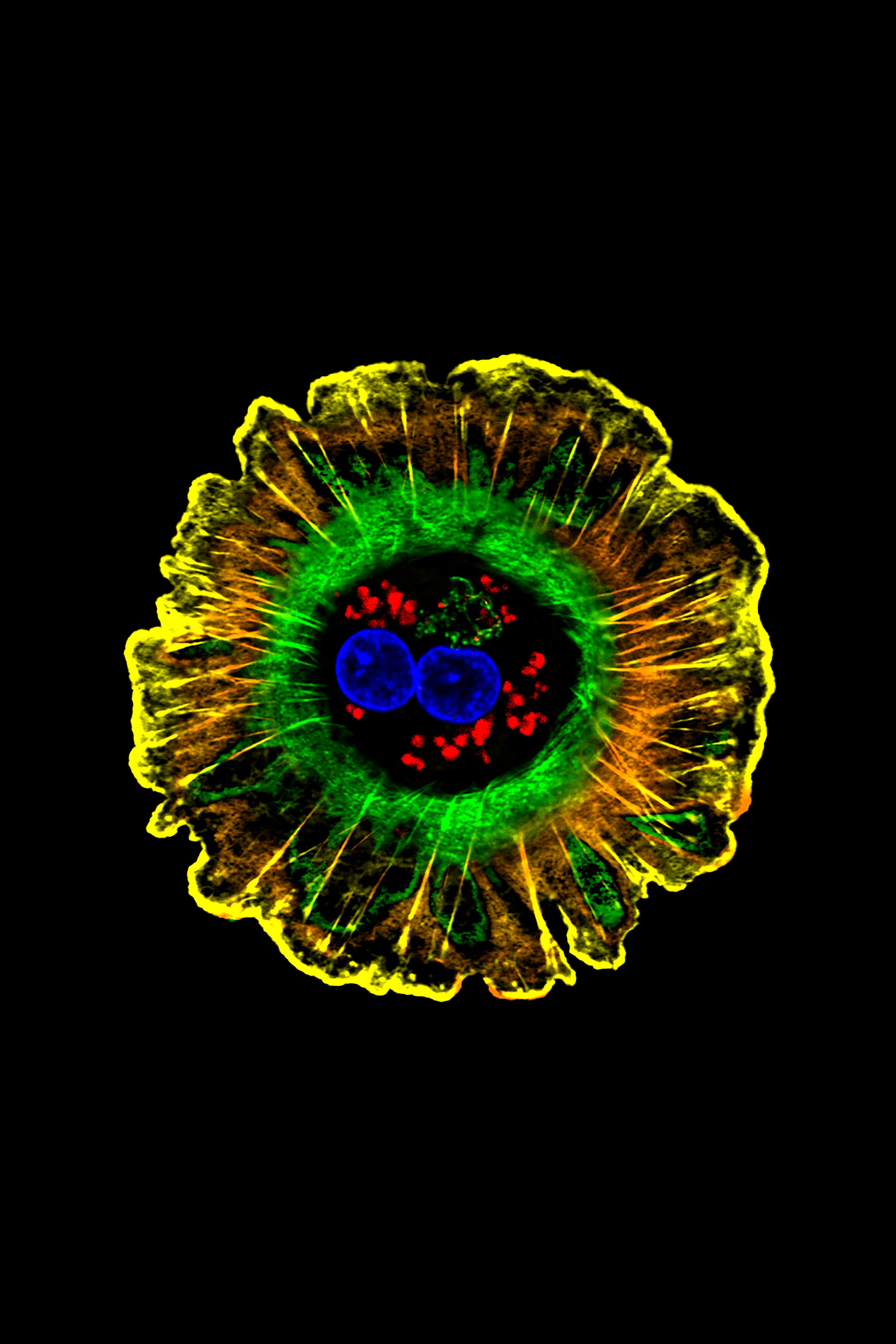
Liver cell
This is not biology's first attempt at an ambitious, "man-on-the-moon" project, and past initiatives have proven tremendously beneficial to science. The impact of the Human Genome Project (HGP) cannot be understated, for example: it has accelerated the rise of new fields, like synthetic biology, and enabled gene therapy and personalized medicine. The Human Protein Atlas, started in 2003, has now mapped over 12,000 proteins in more than 30 sub-cellular compartments, providing fundamental knowledge about the proteins that drive our cells' daily functions. Mapping cells is the next logical step, to discover new cells – a feat the HCA is already touting – and to inform new medicine and treatments. As cell and gene therapies advance, we will need a full toolbox of cell types in easy reach.
Smoothies vs fruit salad
The project arose at the perfect time, five years after the technology, termed single-cell genomics, became well-established. Single-cell genomics allows researchers to measure how individual cells are expressing their genes. To put the power of single-cell genomics into perspective, I will borrow an analogy used by Regev, comparing a common smoothie to a fruit salad. In previous sequencing methods, cells extracted from tissue were mixed and the end results represented an average of that mix: the smoothie. But just as you won't likely taste one blueberry in a strawberry smoothie, rare cells can easily be lost in the mix of common cells.

A mixed metaphor
However, with new single-cell techniques, the results resemble a fruit salad – each and every fruit can be seen and tasted, even if there are 10 strawberries to one blueberry. These rare cells (the metaphorical blueberries), which often have specific and crucial tasks, can now be seen and understood in the greater picture of different tissues. Researchers have quickly adopted these techniques and are developing the computational methods to understand their results, giving the Human Cell Atlas the technological foundation to succeed.
While researchers have adopted this technology en masse in the last few years, many researchers argue that the field is still learning how to use it. Just as the Human Genome Project, with its huge funding siphon, has catalyzed the explosion of genomic sequencing, the Human Cell Atlas will likely have a similar effect on new single cell technologies. While this has obvious consequences in biology and medicine, like early diagnosis of small cancer populations, it has far-reaching implications for all fields that study life. The technology offers new capabilities to understand the function and development of life, down to each individual building block.
Moonshot problems
While research has already started in this direction, the Human Cell Atlas offers several benefits, including directed and increased funding; open-source data sharing; and improved reproducibility, thanks to standardized methods. They are not without flaws, which have been raised by many people in the scientific community. Big projects, like the Human Genome Project, Human Protein Atlas, Cancer Moonshot, and the BRAIN Initiative, cost millions of dollars and research hours, they note, and while there are valuable discoveries produced from these roadmaps, moonshots, and frameworks, skeptics worry that these projects divert funding and brain power from more creative endeavors aimed at similar goals.
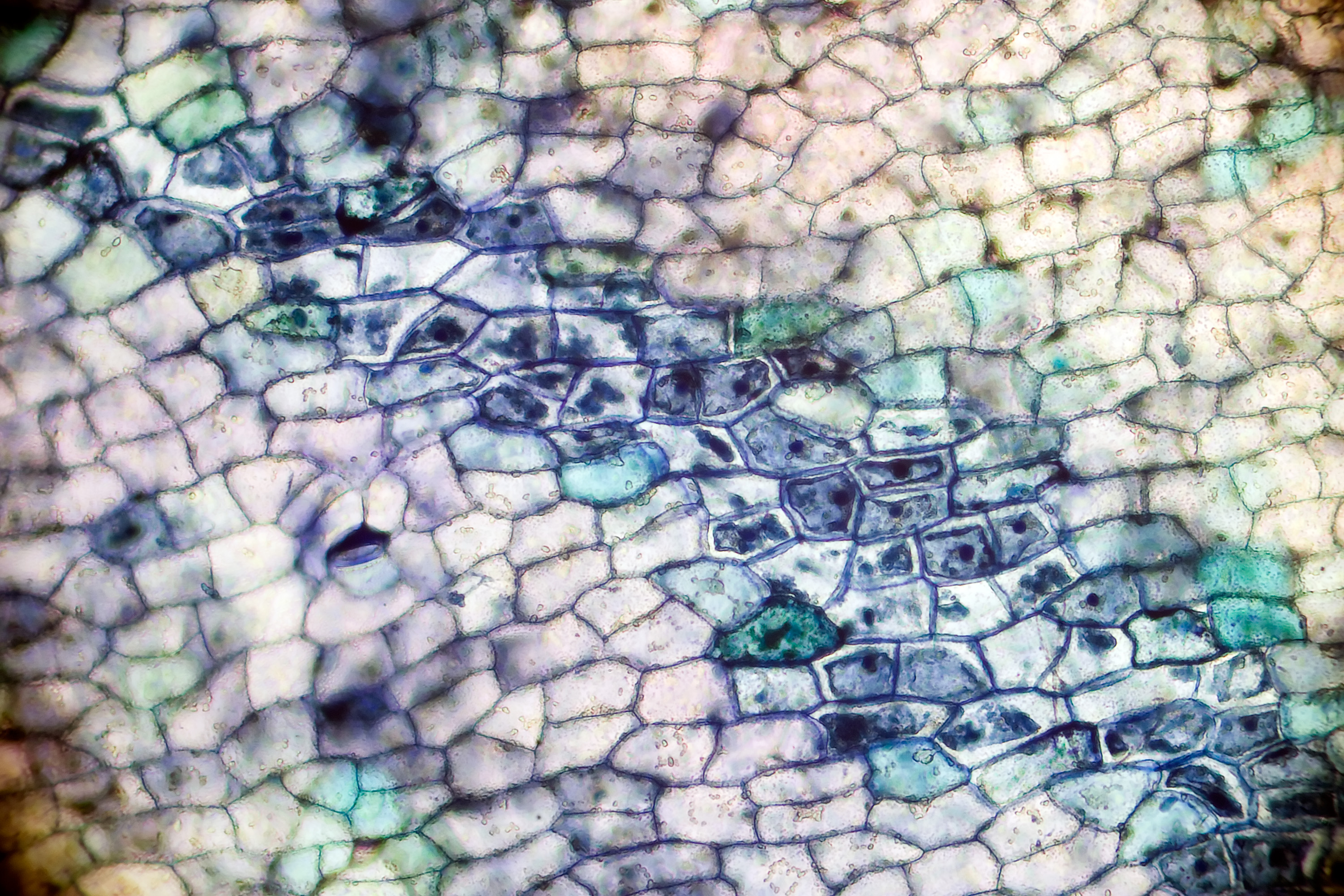
Not human cells (magnolia petals)
They ask a fair question: what is the distinct, specific goal of the Human Cell Atlas? It's undeniable that mapping every cell in the human body would be a spectacular achievement, to put it mildly. But to achieve that goal we must define what makes a human cell – a definition that will surely change as we learn more – and a definition that raises even more questions.
Do we want to know which genes are expressed in which cells? Or how each cell responds when certain genes are knocked out? Without clear guidelines, trying to define a cell can quickly feel like an impossible game of 20 Questions, and while the HCA has laid out a loose framework in its initial whitepaper, it leaves much to address as the first rounds of data roll in.
Lastly, the research needs to confront the issue of diversity, which is nonexistent by some estimates. This issue is particularly prevalent in genome-wide association studies (GWAS), in which thousands of individual genomes are scanned for variations that correlate with specific traits, like a disease. Past studies have missed key variants due to dominating ethnicities, and we can expect the same from the Human Cell Atlas if researchers fail to acquire diverse samples.
Small changes in genes across populations can cause significant changes in height. Hundreds of variants of specific genes can significantly alter how people respond to cancer therapies. These small variations pile up, so cell diversity will be crucial to producing quality data; we will need that quality data to decide an accurate definition, whatever that may be, of certain cell types. While the organizing committee outlined specific guidelines to gather ethnically and geographically diverse samples, the first draft explicitly states that initial data sets will not meet these aims.
It should be of primary concern for the Human Cell Atlas to avoid repeating the mistakes of history. Although less-expensive than the Human Genome Project, the Human Genome Diversity Project (HGDP) has failed by most measures, inching along with disjointed support, years after the HGP wrapped up. Perhaps by integrating diversity requirements into their framework, the Human Cell Atlas will avoid the mistakes of the HGP and HGDP. But they must overcome the continuing problem of sample collection, which inherently costs more when it aims to achieve more diversity and better results.
The Human Cell Atlas isn't perfect, and faces steep challenges. But it is also an ambitious and much-needed project, led by top geneticists, biologists, clinicians, engineers, and computational scientists across 10 countries. It has all the makings of moonshot project, poised to create a map of how you and I develop from a single cell. We will have to watch as data are produced and the Atlas further defined, urging the scientists to hear our concerns, and encouraging them in awe as this international consortium attempts to map each and every cell.