Pulling all-nighters may damage your brain
New research suggests bad sleep causes a build-up of plaque associated with Alzheimer's
For decades, scientists have suspected that there is a connection between poor sleep and neurodegenerative diseases like Alzheimer’s disease. People with Alzheimer's often have low quality sleep, and the same thing has been found in animals with the disease. Similarly, older adults who slept for less time, or had poor quality sleep, had higher levels of beta amyloid, a plaque-forming protein linked to Alzheimer's disease, in the brain.
To date, however, scientists have been uncertain whether poor sleep contributed to the development of Alzheimer's or if poor sleep was simply another symptom of the disease. In other words, does bad sleep cause a buildup of beta amyloid or does too much beta amyloid cause people to sleep badly?
Sleep risks and Alzheimer's
A study recently published in the Proceedings of the National Academy of Sciences (PNAS) may reveal the answer. In this study, researchers kept people awake all night and measured levels of beta amyloid in the participants’ brains the next day. They then compared these levels to the beta amyloid levels in the same subjects’ brains after they had had a full night of sleep. Researchers found that a single night without sleep causes an increase in the amount of beta amyloid in the human brain. This indicates that poor sleep is likely an important risk factor for the development of Alzheimer's.
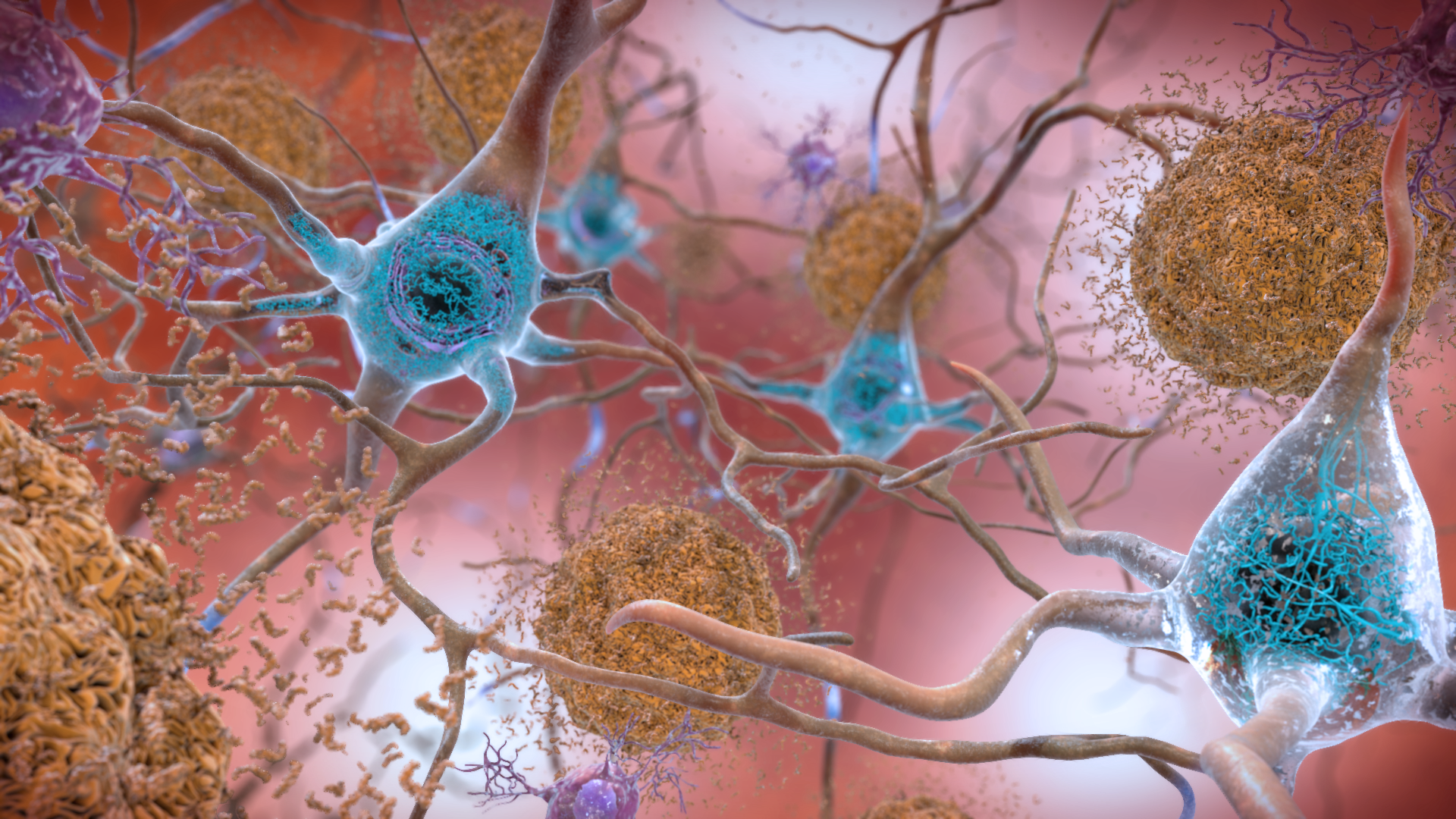
Beta amyloid and Tau
NIH Image Gallery / flickr
So how were scientists in this study able to measure levels of beta amyloid, a microscopic protein fragment, in the brains of living humans? When doctors need to measure proteins in other tissues like skin, muscles, liver, etc., they simply biopsy a tiny piece of the tissue, usually with little risk to the patient. However, the brain is extremely sensitive, so it is very dangerous to perform a brain biopsy. Any bleeding or swelling in the brain could cause the patient to have a seizure or go into a coma, so a brain biopsy would never be allowed in an experiment. Brain biopsies are generally only used when doctors suspect the patient has a life-threatening condition. So for much of history, scientists who wanted to study proteins in the human brain needed to wait for someone to die and donate their brain to science.
But in recent years, scientists have developed new techniques enabling them to peer inside the living human brain. In this study, researchers used positron emission tomography, also called a PET scan. Researchers injected participants with a special radioactive dye called 18F-florbetaben (FBB). FBB has been designed to stick to beta amyloid, so when FBB enters the brain, it sticks to the beta amyloid floating around. FBB emits a tiny amount of radiation, which is detected by the PET scanner. By determining how much radiation there is and where it's coming from, scientists can tell how much FBB is in the brain and, by extension, how much beta amyloid there is. Although PET scans do expose participants to some radiation, it is generally considered low-risk: about 2 million PET scans are performed every year in the US alone.
This study, led by researchers from the National Institutes of Health, found that in certain brain areas, beta amyloid levels increased after just one night without sleep. Researchers were particularly interested in the hippocampus, a brain area that is essential for memory and is often damaged in people with Alzheimer's. Like many other body parts (eyes, hands, feet, lungs, etc.), we have one hippocampus on each side of our body. Weirdly, scientists found that sleep deprivation significantly increased beta amyloid levels in the right hippocampus, but not the left one.
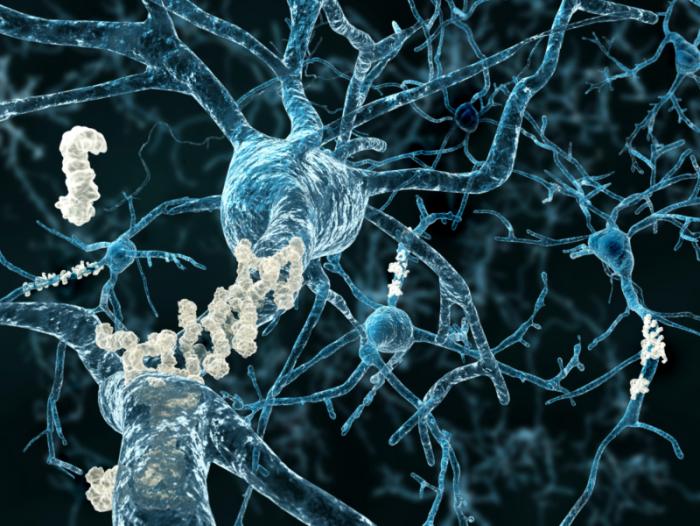
Beta amyloid plaques
vestque / Flickr
Although the left and right hippocampi play relatively similar roles in memory processes, there is some evidence that the left hippocampus may be involved in autobiographical memory (events from our own lives), whereas the right hippocampus seems to be slightly more involved in spatial memory. Researchers were unable to determine exactly why there was such a difference in beta amyloid between the left and right sides; it’s something that will have to be explored in future studies.
In addition, the researchers only measured beta amyloid levels in the afternoon after a night of sleep deprivation, so they weren't able to determine how long beta amyloid levels remained high. Future research will have to examine if beta amyloid levels return to normal after a night of good sleep or if the effects of an all-nighter persist for longer lengths of time.
Mysteries and limits
Another important question to investigate is why exactly sleep deprivation leads to more beta amyloid in the brain. Although this experiment didn’t attempt to determine mechanisms, a previous study in mice found that sleep deprivation resulted in high levels of beta amyloid in the brain.
Researchers then wanted to know if sleep deprivation caused beta amyloid to be produced faster or if the awake brain just wasn't as good at getting rid of the extra beta amyloid. To find out, a different group of researchers injected beta amyloid into a mouse's brain and then measured how quickly it got cleared away.
They found that beta amyloid was cleared from the brain more efficiently during sleep than during wakefulness. Brain cells are bathed in fluid, which circulates throughout the brain to help clear away potentially toxic proteins. During sleep, the space between brain cells increases. This increased space may allow the fluid to flow better, improving its ability to wash proteins like beta amyloid out of the brain.
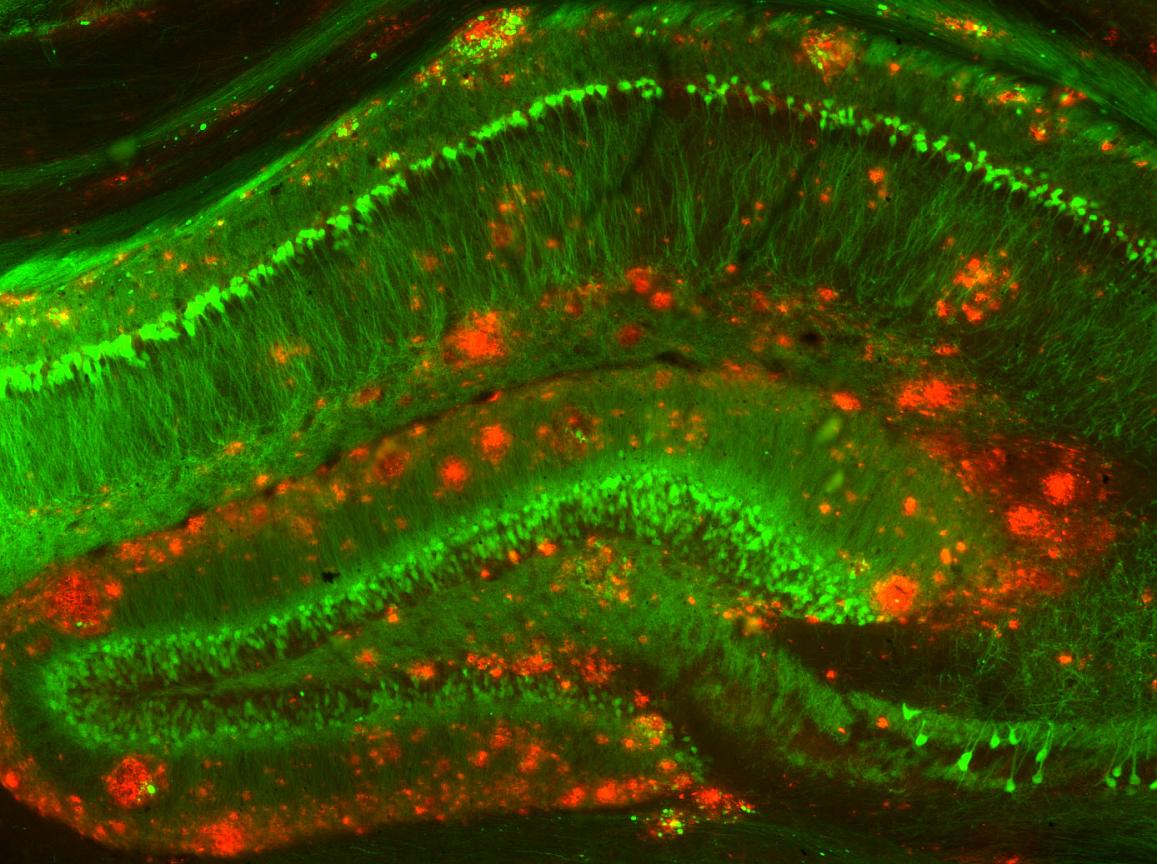
Mouse model of beta amyloid
NIH Image Gallery / Flickr
Another big mystery is the variability in responses to sleep deprivation. On average, sleep deprivation increased brain beta amyloid; however, some participants had very small increases in beta amyloid, while others had much larger increases. Researchers were unable to figure out why this was. The increase in beta amyloid didn’t appear to be associated with the participant’s gender or age or whether they had a certain genetic risk factor for Alzheimer's (a specific mutation in a gene called APOE).
This study used a fairly small sample size (20 people) and collected limited medical and genetic background data on the participants. Using a larger sample size and collecting more data about the participants may allow future researchers to determine factors that influence why some people have large beta amyloid increases, while others seem to be less susceptible to the effects of sleep deprivation.
Exploring the link between sleep and Alzheimer's is important, because poor sleep may be one of the few risk factors for Alzheimer's that individuals can control. Other known risk factors include being female, being Latino or African-American, and having a mutated form of the APOE gene. Future generations may be able to use gene editing to correct mutated APOE genes, but for now, our genes aren’t something that we can control. But we can change our behaviors.




Wish I’d been told before thesis writing ☠️