.jpg?auto=compress%2Cformat&crop=faces&fit=crop&fm=jpg&h=360&q=70&w=540
)
Anna Andersen
Who decides when life begins? How do we define a disease? What kind of gene edits should be allowed?
Who has the answers to the questions posed by genetic modification?
New Lab’s quarterly event series, Existential Medicine, explores technological disruption in the medical field and its impact on the human experience. In part three, The Genetic Horizon, panelists discuss the ethical implications of CRISPR-Cas9 and how willing we should be to augment our DNA.
Scientists had been trying to develop easy and precise gene-editing tools in the lab for years. But they found a solution already existed in nature when a yogurt company discovered CRISPR in its bacteria, 12 years ago.
Today, twin girls have allegedly had their genome altered by CRISPR-Cas9. Since altering even one gene during development can have unknown consequences once the child is born, the world condemned Chinese doctor He JianKui who claims to have embarked on a rogue experiment to make these babies HIV resistant.
Researchers worldwide had long agreed to not cross a hard redline: instructing genetic changes to germline cells, which would pass through lineage and onto other generations. Thus, He’s news has created urgency to identify an international rulebook and surfaced existential concerns around morality and governance. Who decides when life begins, how do we define a disease, and what kinds of genetic modifications should be allowed, or even encouraged? These are not questions for a single entity to answer, but demand the attention of an entire society; from pharma and disability rights, to science, academia, and technology — the list goes on.
Nature’s Solution
At the third Existential Medicine, moderator Janna Levin, the Tow Professor of Physics and Astronomy at Barnard College opened the evening: “I have been absolutely mesmerized by the biology, physics of its mechanism, and the promise of CRISPR, but also terrified of what might go wrong.” She’s not alone.
Similar to how humans have evolved the immune system to fight off pathogens, bacteria have developed defense mechanisms against invading viruses. These viruses replicate by inserting their genes into the bacterium; the bacterium is then able to recognize the viral DNA as foreign genetic material and trigger a protein that acts like molecular scissors to unzip the DNA and inactivate the virus. The precision by which this molecular strategy could detect a chunk of DNA was stunning to scientists.
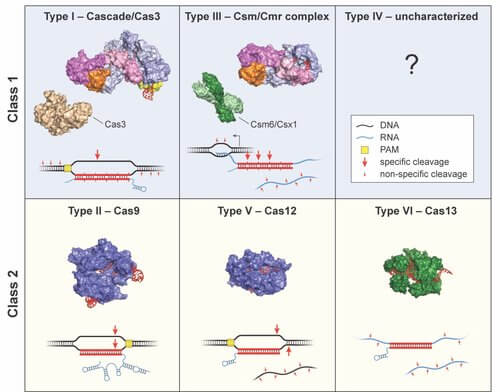
CRISPR–Cas systems are remarkably diverse. Sternberg Labs has shown that many of the same strategies Cas9 uses during target interrogation are similarly exploited by Type I systems. They continue to explore other immune system subtypes and their potential for genome engineering applications.
They realized the same approach could be repurposed into a tool to zoom in on a specific part of the DNA in any organism — from plants to humans — to delete or alter a gene. “CRISPR is basically an elaborate molecular strategy to recognize DNA from an invading virus in a very specific way,” said Samuel Sternberg, head of Sternberg Labs and Assistant Professor of Biochemistry and Molecular Biophysics at Columbia.
Sternberg detailed how the media can paint a different picture than reality. “In a lot of stories, they say CRISPR is this highly precise, amazing gene editing tool. It is actually not that easy in practice,” he said. “If you want to make a therapeutically safe edit, or a mutation, it’s not ready for prime time.” That’s because most genes don’t map to only one function — their effects are combinatorial, largely unknown, and still the focus of intense research. After all, it was only a few decades ago that humans started to read the code of life.
A Molecular Swiss Army Knife
Beyond treatment possibilities, CRISPR has made significant contributions to basic science. “It is being used to gain knowledge at the level and resolution that we could never have imagined before,” said Ali Brivanlou who runs the Stem Cell Biology and Molecular Embryology Lab at The Rockefeller University. His team has developed a system to develop artificial human embryos. “We use CRISPR-Cas9 to identify where the lineages come from, [such as] how a cell inside an embryo decides if she is going to become blood, muscle, brain, and skin,” he said.
During these early stages of embryonic development, humans also develop organs we never imagined existed. “We have gills, a tail, we breathe in water — these are natural attributes. These are not CRISPR-Cas9 changes, the information is in our DNA,” Brivanlou said. “And it’s not impossible to imagine that we can reawaken that information.”
CRISPR is also revolutionizing the ability to identify new biological and disease mechanisms by cutting down the time it takes to study the genes. “CRISPR cuts years of work into days,” said Michal Preminger, Head of East and North America at Johnson & Johnson Innovation, JLABS. What used to be a PhD thesis, can now be done in weeks. “There is a lot we don’t know about biology and the human condition that will unfold, thanks to advancements in science like CRISPR-Cas 9,” Preminger said.
Some teams are even using CRISPR to target pathogens. J&J, for example, recently struck a deal with Locus Biosciences to develop a virus that carries a kind of CRISPR that removes harmful bacteria from the body while leaving beneficial bacteria untouched.
The Genetic Puzzle
At a critical point in time — and in a rapidly changing field — He’s uncorroborated claims have led to meaningful dialogue around misuse, where we go from here, and what kinds of interventions are justified. In the United States, for example, the scientific community is regulating itself. But at an international scale, it’s not up to scientists alone to decide how the use of CRISPR evolves.
Considering the potential of this technology, all guest speakers unanimously agreed there is an immediate need to open this conversation to all facets of our society. “At the end of the day, it comes back to the same question: the origin of life and of human beings,” said Brivanlou. “When do we consider an embryo to be a human and deserve recognition of moral status? These are questions that are dealt with differently in different cultures and religions.” Initiating the debate with considerations to the multicultural fabric of our society is arguably more important than the outcome, Brivanlou described.
While concrete answers are not yet in sight, it is clear the ethical considerations can go both ways. As Brivanlou said, “We can agree: if you can correct something that otherwise would not allow a child to die, then you can ask yourself: if I have a technology to save lives and I don’t use it – is that really ethical?”
Existential Medicine is a quarterly series presented by New Lab and Johnson & Johnson Innovation, JLABS, with supporting partner EY.

