
www.ccPixs.com
A failed Alzheimer's drug stops cancer cells from shedding their coats and hiding from the immune system
A chance reading and a fortuitous overlap between Alzheimer's and multiple myeloma biology gave a failed drug new life
In the pharmaceutical industry, failure may be just the beginning.
Imagine spending over 10 years and millions of dollars only to have a promising drug candidate fail a Phase III clinical trial. That’s what happened to the drug companies Eli Lilly in 2010 and Bristol-Myers Squibb in 2012. Phase III is usually the final barrier before Food and Drug Administration (FDA) approval, so it is especially disappointing for drug candidates to fail at this point.
Both failed drugs are members of a class of compounds called gamma secretase inhibitors (GSIs), intended to treat Alzheimer’s disease. β-amyloid peptide (Aβ) accumulation in the brain is a hallmark of Alzheimer’s disease. Scientists and drug companies once believed that inhibiting gamma secretase, a protein known to produce Aβ, could cure the disease. The GSIs passed Phase I trials and Eli Lilly's made it to Phase III. Failure of GSI drugs that were so close to approval was devastating.
Maybe now the blow will be softened. Stanley R. Riddell and Damian J. Green are scientists at the Fred Hutchinson Cancer Research Center in Seattle. Riddell is a world leader in the development of immunotherapy, treatments aimed at charging up a patient’s own immune system to attack tumors and Green is a myeloma specialist. Riddell and Green focus on a specific type of immunotherapy called CAR-T, where the patient's own T-cells are modified to specifically target a particular protein from a particular cancer type. Seven years after the Phase III failures of GSIs, Riddell and Green have come up with a new approach to treating cancer using these GSIs that failed to help people with Alzheimer's.
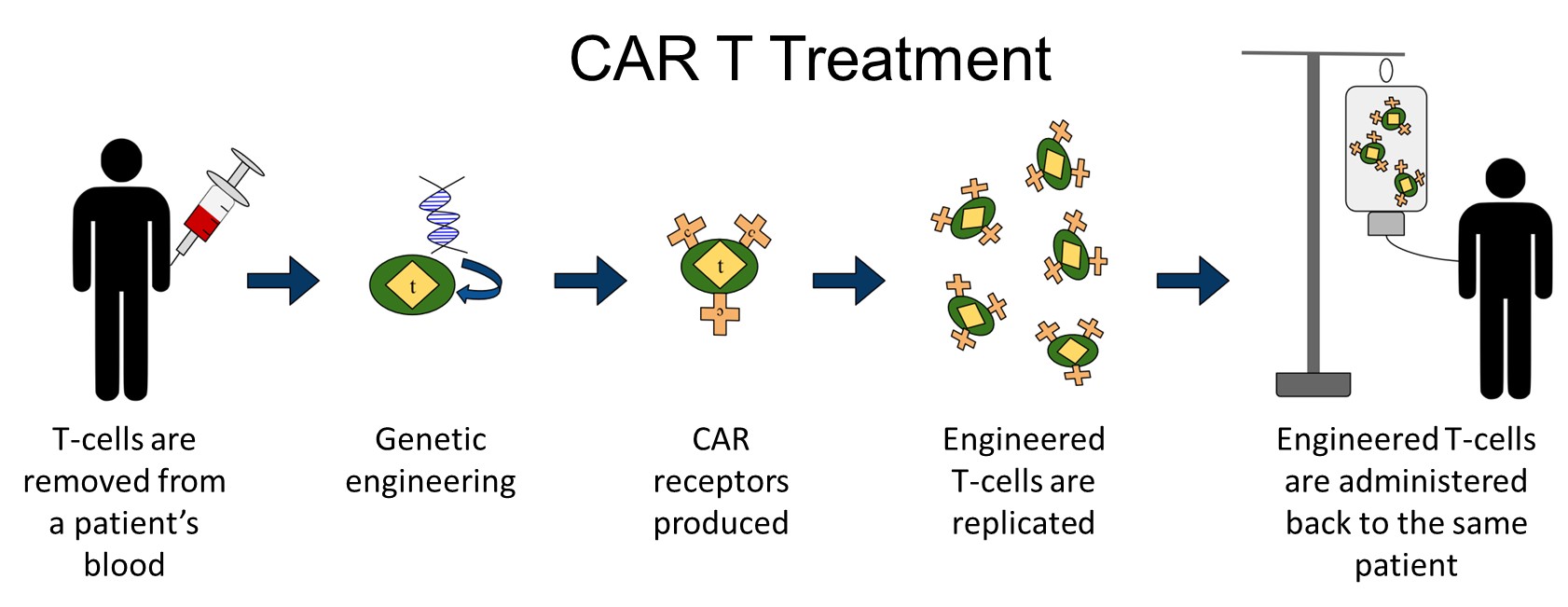
CAR T is a type of immunotherapy in which the patient's own immune cells are modified to better fight cancer.
Reyasingh56 via Wikimedia Commons
Riddell and Green wanted to use CAR-T to target and destroy multiple myeloma, a cancer of blood plasma. CAR-T has been successful in some clinical trials for certain cancer types but as of 2019 the FDA has only approved a handful of CAR-T therapies, prescribed only for patients with certain blood cancers after other treatments have failed. Riddell and Green were faced with the specific difficulty of dealing with multiple myeloma's ability to hide itself from the immune system.
For their CAR-T's multiple myeloma-specific target, Riddell and Green chose B-cell maturation antigen (BCMA), a protein found on the surface of multiple myeloma cells. However, multiple myeloma seems to know that BCMA is a giant red flag. Multiple myeloma cells can shed BCMA like a dog shedding fur, effectively hiding themselves from immune responses, including CAR-T cells.
Riddell and Green found themselves with a serious challenge – if the immunotherapy couldn’t "see" the cancer, their treatment wouldn’t be effective. This is where the research stood until Green picked up a passing reference about the failed Alzheimer’s trials and how GSIs seemed to impact the amount of BCMA present on the cell surface.
Killer T cells surround a cancer cell
Alex Ritter, Jennifer Lippincott Schwartz and Gillian Griffiths, National Institutes of Health
It was once thought that that inhibiting gamma secretase, a protein known to produce Aβ, would be an effective treatment for Alzheimer's disease. But clinical trials of GSIs for treating Alzheimer's were halted when it came to light that treatment actually made the disease worse. It caused more instances of side effects, including skin cancers and gastrointestinal toxicity, and worsened cognition and function compared with Alzheimer’s patients receiving standard treatment. After these trials were terminated, the scientific community shied away from GSIs for Alzheimer’s treatment, as it seemed like the potential benefits did not outweigh the risks.
But it turns out that gamma secretase doesn't just form Aβ in the brain, it also removes BCMA from cancer cells. Could inhibiting gamma secretase force multiple myeloma cells to hold onto BCMA and make them better targets for immunotherapy?
In a recent article published in the journal Blood, Riddell, Green and their collaborators at the Fred Hutchinson Cancer Research Center, the University of Washington, University Hospital, Würzburg, and Eli Lilly and Company, believe they have sufficient evidence to support the combined use of GSIs (to keep cancer cells visible) and BCMA CAR-T (to kill the tumors.)
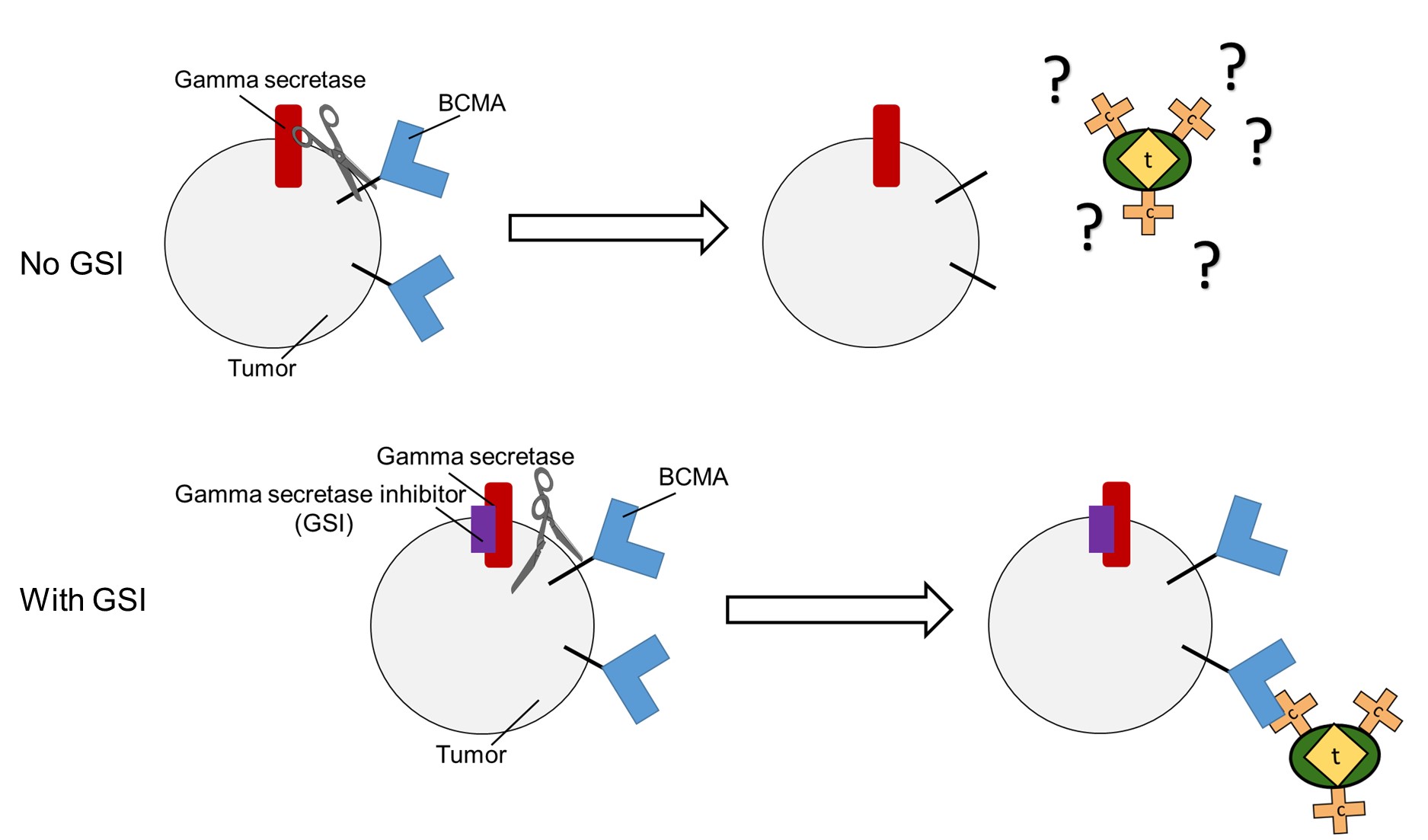
Gamma secretase sheds BCMA from tumor cells, hiding the cancer from CAR-T therapy. Riddell and Green hope to use a class of failed Alzheimer's drugs, GSIs, to keep the cancer visible.
Christina M. Marvin
The team knew that BCMA CAR-T therapy showed very low (8 percent – 39 percent) response rates in multiple myeloma patients. Unfortunately, very little research was available to suggest a reason for the ineffectiveness. Using cells grown in the lab and cells derived from multiple myeloma patients, the scientists demonstrated that cancer cells treated with GSI contained more BCMA on their surfaces and that BCMA CAR-T therapy was more effective on the GSI-treated cells.
While these results ultimately support their hypothesis that GSI could improve CAR-T therapy, the scientists found two clinically important caveats. Once the GSI was removed from the system, BCMA levels returned to normal. Secondly, there appeared to be a threshold BCMA limit at which too much BCMA rendered CAR-T therapy ineffective. While these details might seem small in the scope of the paper, they have serious implications for the dosing strategy of GSIs in clinical trials. It appears that doctors must maintain a constant level of GSIs to prevent the BCMA from shedding, but also be careful not to raise the dose too high so that the amount of BCMA prevents CAR-T therapy from working.
In their study, the researchers also included results from a small group of patients. While only encompassing three patients, the preliminary results are impressive: the percentage of multiple myeloma cells with any BCMA on them increased from a median of 27.5 percent to 99.3 percent and the BCMA density on cancer cells increased between 8.7-fold and 157-fold.
Based on these results, a complete Phase I trial for the GSI/CAR-T combination treatment is currently in progress.
While this story is one about a new cancer therapy, it is also a narrative of scientific development. Science should be viewed as a process, constantly building on and correcting itself. Alzheimer's drugs have a history of failing in clinical trials, but this team showed that a drug that fails in one context could be life-saving for patients with a different disease.



There are numerous compounds that have been repurposed or are currently undergoing investigation for indications other than their original intended use: a hypertension drug used for hair loss, a failed morning sickness drug and sedative for multiple myeloma, and the list continues. Christina’s article does an excellent job highlighting two important things: (1) Get out of your comfort zone, attend talks outside of your field, and read things unrelated to what you study; (2) A trial only fails if we do not learn anything from it. We do a disservice to the scientific community, but more importantly, the patient community and research participants, if we do not strive to learn all we can from the data collected and make those findings available.