Ancient plankton have climate data hidden in their shells
Scientists have discovered a new way to use single-celled plankton to estimate large-scale changes in ocean chemistry
The fundamental laws that govern the nature of matter haven't changed since the formation of the earth. Since chemistry and biology still operate by the same rules, fossil records can provide important lessons about how earth’s climate changed in the past. For example, small changes to the chemistry of coral skeletons can indicate when earth's temperature might have changed before humans were around to observe it. As the earth continues to warm, studying these records can give us some insight into where we're heading.
Jenny Roberts, a postdoctoral researcher at the Alfred Wegener Institute in Bremerhaven, Germany, is working on fossil records like these. Roberts and her collaborators recently published an article claiming that lithium in foraminifera shells can be used to calculate the pH of past oceans.
Foraminifera, or "forams" for short, are single-celled plankton that live in the ocean. There are two types: planktic forams, which live in the surface ocean, and benthic forams, which live on the sea floor. Both types of forams make their shells out of calcium carbonate, and most often out of calcite. Calcite is a hard mineral (it's chemically similar to limestone), so when forams die, their shells frequently end up fossilized in the sediments. This—and the fact that forams live all over the world—make their shells some of the most valuable tools used by scientists who study past climates.
Whoa, glad this is microscopic
Generally, researchers try to relate some property of the shell to an environmental condition. Roberts and her lab, for instance, examined the chemical composition of the shells. Calcite is a crystal, which means that its molecules are arranged in what’s called a mineral lattice, a regular structure of calcium atoms connected to each other, like a chain-link fence. When calcite forms, impurities often get caught in the gaps, or end up replacing the calcium atom, in a process known as substitution.
Chemical reactions are often dependent on things like temperature and pH. Because the accumulation of impurities in calcite is, on some level, a chemical reaction—where dissolved materials are being converted into a solid phase—researchers can deduce things like temperature, pH, and the chemistry of the ocean based on the amount of impurities in the shells and how those impurities interact with each other.

Probably chock-full of forams
Isotopes, other types of atoms, complicate the picture even further. Atoms are composed of protons, neutrons, and electrons. Normally, an atom has an equal number of protons and electrons, but an atom of the same element can have different numbers of neutrons. For example, the most common kind of carbon atom has 6 protons and 6 neutrons. This form of carbon is sometimes called carbon-12, or 12C, named after the sum of the number of protons and neutrons. But a carbon atom can have 6 protons and 7 neutrons. In that case, the atom is named carbon-13, or 13C. 12C and 13C are different isotopes of carbon. Both of these isotopes are stable, meaning they won't decay with time.
Lithium, an element that can be found in forams, has two stable isotopes: lithium-7, the common form, and lithium-6, a rare form. Roberts used lithium isotopes to calculate pH in what's known as a culture study; she and her team grew benthic forams in a lab with seawater at an altered pH. As the seawater became more basic, the amount of lithium-7 in their shells decreased. This correlation was strong enough that the researchers were able to accurately calculate the pH of seawater based on the shells' isotopes. Next, the researchers went back to look at old foram fossils, using their lithium isotopes to look back in time and calculate past ocean acidity. Using sediment core samples and lithium-7, they calculated past ocean pH as far back as 25,000 years ago.
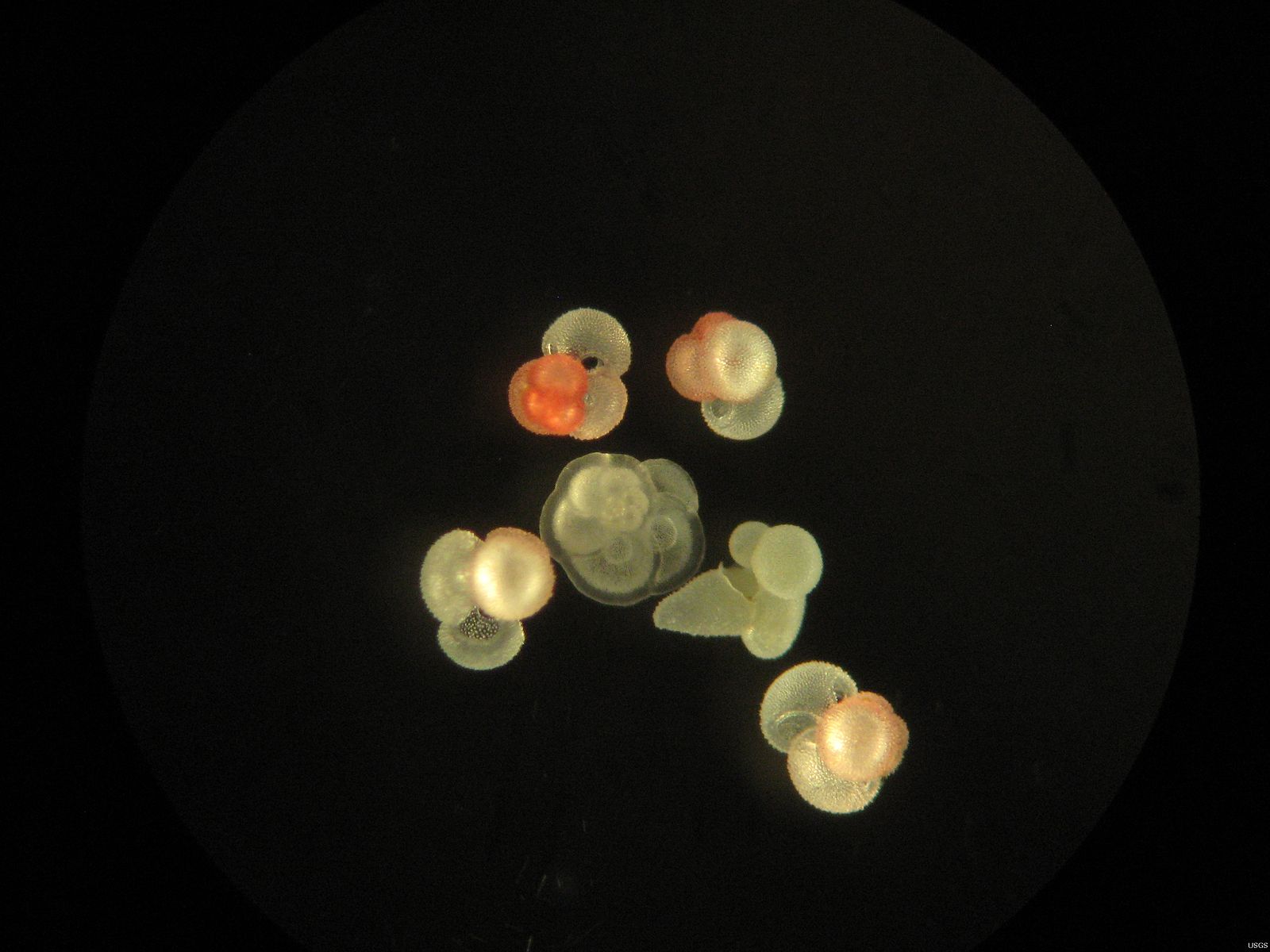
Worth their weight in gold...if you're a paleoceanographer
Of course, because biology doesn't always do what we'd expect, there are often road blocks and new questions that pop up along the way. In this case, while Roberts found these isotope correlations could be applied to fossil records, her estimated pH differed from results collected using another method of calculating pH from fossils. That method focused on boron-11, a different element, in shell calcite. Further studies will be needed to understand these differences.
Furthermore, while 25,000 years sounds like a lot, on a geologic scale, it's really not that long—some of the most dramatic climate changes occurred hundreds of thousands to millions of years ago. New research will likely try to find what caused these observed trends, where different methods of calculating pH can be applied, and what conditions might affect or disrupt these equations.
But overall, it's an exciting glimpse into some of the biggest questions we have about the past. The development of a new proxy for calculating ocean acidity gives us a new tool to study past climate changes—and the effect of those changes on the species and ecosystems that existed at the time. The earth has a lot of stories left to tell.
_Mediterranean_Sea.jpg?auto=compress%2Cformat&crop=faces&fit=crop&fm=jpg&h=360&q=70&w=540
)


So cool! I like that lithium-isotopes are being realized as potential proxies in paleoclimate. They’re gaining popularity in the speleothem community, too!
For speleothem-based proxies, we sometimes make the assumption that some parameters haven’t changed, but a lot of new research is aimed at figuring if those parameters actually are significantly variable. With biological proxies, I’ve always wondered how safe of an assumption is it that the biological process hasn’t changed significantly through time. Are other ways to determine if the forams biology has changed?
Do the forams take in less lithum-7 because for some biological reason, or is it because lithium-7 abundance in seawater is pH dependent so there is just more or less lithium-7 available for the foram?
I like that they are comparing it to boron-isotopes! How large of a disparity did they see between the lithium- and boron-isotopes? And were the long-term trends the same, or did they completely deviate?